What is austenitic stainless steel, martensitic stainless steel,alloy steel?
What is austenitic stainless steel? What is martensitic stainless steel? What is alloy steel? Here you can find the answer.
In mechanical design, we often use austenitic stainless steel and martensitic stainless steel because they have good physical and mechanical properties.
For example: the commonly used austenitic stainless steels AISI303 and AISI304 have an elastic modulus of around 200 and yield strength of 190Mpa-230Mpa.
Commonly used martensitic stainless steels AISI420 and AISI440C have an elastic modulus of 215Gpa. After 420 quenching and tempering heat treatment, the yield strength can reach 345Mpa-1420Mpa. After 440C heat treatment, the yield strength can even reach 1900Mpa.
Quenching is the process of heating the workpiece to 30-50°C above the critical temperature of austenitization, taking it out after insulation, and cooling it rapidly in water.
Tempering is the process of reheating the quenched workpiece to below 727°C, taking it out after insulation, and cooling it in air, oil, or water. Generally, tempering is required after quenching to eliminate internal stress and stabilize the structure.
Austenitic stainless steel is non-magnetic and has good corrosion resistance, such as 303, 304, 316, 202, and other stainless steel.
Martensitic stainless steel is magnetic, but its corrosion resistance is not as good as austenitic stainless steel, such as 420, 440, 410, 403, and other stainless steel.
Whether it is martensite or austenite, they are essentially formed based on pure iron with different concentrations of carbon added at a certain temperature.
When pure iron is heated above its melting point of 1538 degrees, pure iron becomes liquid.
When pure iron begins to cool in a liquid state, it will crystallize into crystals with different structures in different temperature ranges. (Crystal refers to the change of liquid into solid; crystal refers to an object whose atoms are regularly arranged in space.)
For example, between the melting point and 1394 degrees, iron crystallizes into a body-centered cubic structure, called δ-Fe. Between 1394 and 912 degrees, iron crystallizes into a face-centered cubic structure, called γ-Fe. When the temperature drops below 912 degrees, also has a body-centered cubic structure, called α-Fe.
Iron in the above three temperature ranges, δ-Fe, γ-Fe, and α-Fe, can also dissolve carbon, but their ability to dissolve carbon is different. This is called a solid solution.
Carbon dissolved in α-Fe is called ferrite Ferrite=F, and it still maintains the body-centered cubic structure. Carbon dissolved in γ-Fe is called austenite Austenite=Au, which still has a face-centered cubic structure, and austenite has very good plasticity. ,easily transformed.

However, because the atomic gap of γ-Fe is larger than that of α-Fe, the carbon concentration it can dissolve is larger than that of α-Fe.
The maximum carbon dissolved in austenite is 2.11%, and the maximum dissolved carbon in ferrite is 0.0218%.
What happens if the mass fraction of carbon exceeds the solubility limit of both?
The compound Fe3C will be formed, called cementite: Cementite and its carbon content can reach 6.69%.
When the temperature is lower than 727 degrees, austenite will mix with other structures to form new structures, and most of the stainless steel we usually use is at room temperature. At normal temperatures, the structures formed by different concentrations of carbon dissolved in iron are different.
For example, when the carbon content is less than 0.0218%, the structure formed at room temperature is ferrite.
So, where does austenitic stainless steel come from? What is an alloy?
Carbon steel is an alloy with iron and carbon as its main components. An iron-carbon alloy with a carbon mass fraction of 0.0218%-2.11% is called steel. Among them, carbon steel with a carbon content of less than 0.25% is called low-carbon steel. Carbon steel with a carbon content of 0.25%-0.6% is also called medium carbon steel. When the carbon content is greater than 0.6%, it is called high-carbon steel.
At room temperature, carbon steel with different mass fractions will form austenite when heated above the critical temperature. This austenite has a characteristic that it is isothermal in different temperature ranges, or cools at different times. Cooling at low speed will form different structures.
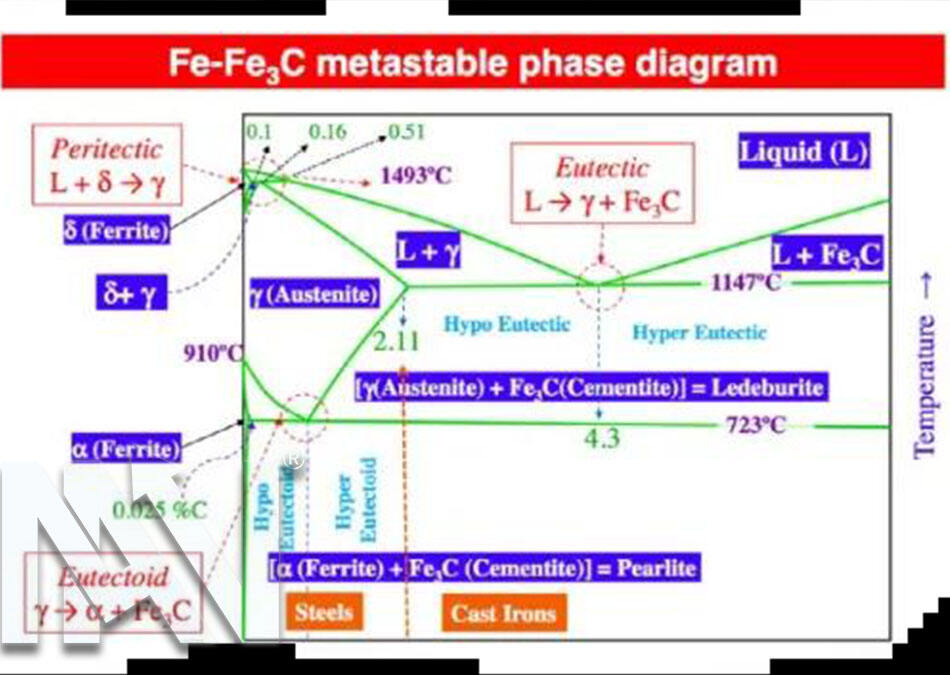
The critical temperature is the temperature corresponding to the A3, Acm, and A1 lines in the iron-carbon phase diagram. It represents the temperature at which different mass fractions of carbon begin to transform into austenite when heated. For example, carbon steel with a pearlite structure at room temperature will be heated to 727 degrees, austenite begins to form.
For example, for carbon steel with a carbon content of 0.77%, pearlite will be formed isothermally between the critical temperature of 727 degrees and 560 degrees, bainite will be formed isothermally between 560 degrees and Ms, and between Ms-Mf Martensite is formed isothermally.
An alloy refers to a metal element combined with other elements to form a substance with metallic properties.
For example, the aluminum alloy windows in your home are an alloy composed of aluminum, magnesium, and silicon. The main body of the kitchen faucet is generally a copper alloy, mainly copper and zinc, and also contains a small amount of lead.
Lithium-aluminum alloy AL-Li8090 and titanium alloys are often used in aircraft structures because of their large ratio of strength to density.
 EN
EN
 AR
AR
 FR
FR
 DE
DE
 RU
RU
 ES
ES
 ID
ID

